UW scientists grow neurons that integrate into brain
Scientists at the University of Wisconsin-Madison have grown human embryonic stem cells into neurons that appear capable of adapting themselves to the brain’s machinery by sending and receiving messages from other cells, raising hopes that medicine may one day use this tool to treat patients with such disorders as Parkinson’s and amyotrophic lateral sclerosis, commonly known as Lou Gehrig’s disease.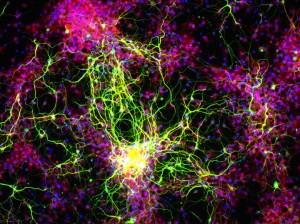
Researchers inserted the human cells into the brains of mice where they successfully integrated themselves into the wiring. Then the UW team applied a new technology, using light to stimulate the human cells and watching as they in turn activated mouse brain cells.
In a lab dish, the brain cells or neurons began firing simultaneously “like a power surge lighting up a building,” said Jason Weick, an assistant scientist at UW who worked on the study published online Monday in the journal Proceedings of the National Academy of Sciences.
Weick said the use of light stimulation, called optogenetics, raises the possibility of modifying transplanted brain cells, in effect turning them up or down like the dimmer control on a light.
“You can imagine that if the transplanted cells don’t behave as they should, you could use this system to modulate them using light,” said Su-Chun Zhang, a UW professor of neuroscience and one of the authors of the new study.
For years, scientists have talked of the possibility of growing neurons in a dish to replace damaged cells in the brain, but there always have been questions about whether the transplanted cells could become fully functional.
But the new work at UW suggests the idea may be poised to make the transition from theory to reality.
‘Function of neurons’
“They have shown real function of neurons. This means they really can play a role in neural repair,” said Arshak Alexanian, an associate professor in the department of neurosurgery at the Medical College of Wisconsin, who did not participate in the UW study.
“We are getting similar results,” Alexanian said, indicating that he has been working along similar lines, only using “neural-like cells” that had been reprogrammed from adult stem cells found in the bone marrow. The advantage to using the reprogrammed adult stem cells is that they would come from the patient, removing the risk of rejection.
When scientists reprogram cells, they change them from one type to another, a trick that can be accomplished through a variety of methods. Alexanian used synthetic molecules to change the cells.
Recent preliminary results from his lab have shown that the human neural-like cells, when grown in culture with human neurons, form connections and behave like the neurons we’re born with.
The Medical College researcher has been transplanting these lab-made human neurons into the injured spinal cords of rats. While the rats experience some regeneration of cells without any treatment at all, Alexanian said the transplanted cells spur significant improvement, allowing rats to move formerly immobile hind legs.
Targeting hippocampus
At UW, Weick said the new paper built on almost three years of lab work and was successful both in a lab dish and in a live mouse. The UW team did much of its work using embryonic stem cells rather than reprogrammed cells, which are believed to be very similar. Although the reprogrammed cells are less controversial, scientists say they have some disadvantages. Weick said the embryonic stem cells are more reliable than the reprogrammed equivalents and can be coaxed into neurons with greater success.
Weick said the UW scientists have repeated some of the experiments using reprogrammed cells, “and it works just fine.”
In the experiments with live mice, the UW researchers anesthetized the animals, inserted a needle into precise areas of the brain and injected the human neurons. The scientists selected a target for the cells where the brain’s architecture is well defined and the cells would have a good chance to integrate into the circuitry: the mouse hippocampus. The hippocampus is the part of the brain in which memories are formed, organized and stored.
When the human neurons were cultured in a lab dish with mouse cortical neurons, the human cells adopted a behavior of the mouse cells called “bursting.” For mouse cells, “bursting” is a kind of rhythmic firing. The cells are, in effect, talking to one another at the same time.


